一些品质、理想和期待是科学家的共识

In classical ballet, artistic creativity is combined with strict discipline. Bacteriologist Emmanuelle Charpentier knows this all too well. Trained in classical ballet and piano, she says it’s not a bad background to have as a researcher citing this as the source of her meticulous accuracy and persistence through repetitive efforts. And, she adds, a scientist needs to cultivate her/his artistic side, be creative and a little bit crazy — at least sometimes.
Leisure: “I have been very busy with work in recent years and even more as a result of all the attention surrounding CRISPR-Cas9, but I really try to keep up with other interests too, such as sporting activities. I am very much interested in culture, art and design. I can at least find the time to listen to music while working, walking and thinking, and I enjoy listening to debates by philosophers and sociologists that question the world and our society. This is where I find my energy and balance.”
Best mode of transport: “Bike! I cycle wherever I am – Paris, New York, Vienna, Umeå, Braunschweig – and currently on a daily basis in Berlin.”
“I was and remain very passionate about the MIMS concept at Umeå University. It focuses on a very high level of education and research, with regard to both high-quality research and insight into how to promote fundamental research and the education of postgraduate students in the long term. The concept also takes into account that good research takes time and requires good working conditions in which a community pools its energy and the administrative burden is small. I appreciate that The Nordic EMBL Partnership for Molecular Medicine gave me the academic freedom I needed.”
可以说,驱动高水平科学家前进的动力,第一位的,永远是求知的渴望和对科学的热情。
“如果我不是个物理学家,可能世界上又会多个钢琴家了!”
虽然没有将音乐作为毕生事业,对于爱因斯坦而言,钢琴可以说是他科学道路上的缪斯女神了。 每当研究进入死胡同时,他就会选择弹钢琴,他说:“我的相对论来源于我的直觉,而音乐是这一直觉背后的驱动力。可以说,我的这项发现来源于对音乐的感知。”
CRISPR-Cas9 – Genetic technology for the future
PROFILE
Emmanuelle Charpentier has received a host of awards from various universities, scientific academies and research foundations and has been included on lists of the world’s most influential people. Researcher Emmanuelle Charpentier’s discoveries are revolutionising genetic engineering. Possible results of her work include new treatments for a range of serious diseases.
Text: Anders Nilsson, Eva-Maria Diehl
In classical ballet, artistic creativity is combined with strict discipline. Bacteriologist Emmanuelle Charpentier knows this all too well. Trained in classical ballet and piano, she says it’s not a bad background to have as a researcher citing this as the source of her meticulous accuracy and persistence through repetitive efforts. And, she adds, a scientist needs to cultivate her/his artistic side, be creative and a little bit crazy — at least sometimes.
Against this background, it seems fitting that the ceremony for the prestigious American Breakthrough Prize for science is more reminiscent of the film world’s Oscars than the austere Nobel Prize Award Ceremony. Together with fellow researcher Jennifer Doudna, Charpentier stepped onstage in 2015 to receive the 2015 Breakthrough Prize from Cameron Diaz.
100 most influential people in the world
The judges of the Breakthrough Prize were not alone in wanting to pay tribute to Charpentier. The same year she was featured in Time Magazine’s list of the 100 most influential people in the world, and academic organisations and journals have showered her research with accolades in recent years. From 2014 and forward she has been awarded a host of awards and prizes: the Swiss Louis-Jeantet Prize for Medicine, the Spanish Princess of Asturias Award, the American Gruber Genetics Prize, the Norwegian Kavli Prize, the Japanese Japan Prize, and the German Leibniz Prize, Ernst Jung Prize for Medicine, Carus-Medal and Hansen Family Award.
Emmanuelle Charpentier
Born: In 1968 in Juvisy-sur-Orge, 20 km south-east of Paris, France.
Position: Scientific, Managing and Founding Director at the Max Planck Unit for the Science of Pathogens, Berlin, Germany. She is also Honorary Professor at the Humboldt-Universität zu Berlin, Germany. Previously group leader at the Helmholtz Centre for Infection Research and Braunschweig/Hannover Medical School in Germany, and visiting professor and group leader at Umeå University. Appointed honorary doctor at Umeå University in 2017.
Leisure: “I have been very busy with work in recent years and even more as a result of all the attention surrounding CRISPR-Cas9, but I really try to keep up with other interests too, such as sporting activities. I am very much interested in culture, art and design. I can at least find the time to listen to music while working, walking and thinking, and I enjoy listening to debates by philosophers and sociologists that question the world and our society. This is where I find my energy and balance.”
Best mode of transport: “Bike! I cycle wherever I am – Paris, New York, Vienna, Umeå, Braunschweig – and currently on a daily basis in Berlin.”
About moving to Umeå: “Perhaps some people wondered how a French city girl would get on here, but I settled in very well. It’s an international environment with researchers and students from around the world, which is something you notice when you go into the city and see what it has to offer culturally. I have also been taken by the nature here, and the snow and dry cold are more enjoyable than the rainy winters in Vienna.”
In 2015, she also received a newly created award from Umeå
University, where she carried out her research from 2009-2015 — the Umeå
University EC Jubilee Award 2015.
“I am honoured and touched to
receive these prestigious awards. This is tremendous recognition for me
and my team, and also for fundamental research in microbiology, genetics
and biochemistry. The awards demonstrate the overwhelming speed at
which our research has been used for biomedical and biotechnological
applications. This sort of support is needed to convince governments and
funding bodies that fundamental research is critical for exploring new
mechanisms, which form the basis for developing new treatment strategies
and biotechnologies.”
The greatest advancements in recent genetic engineering
The reason for all this excitement was that Charpentier’s research at
Umeå University laid the foundation for one of the greatest
advancements in recent genetic engineering. The discovery was presented
in 2012 and
includes the molecular mechanism of an enzyme that has
the not-so-catchy name of ‘CRISPR-Cas9’. Today it’s hard to find a
hotter topic of signifance to so many people in the area of research in
molecular biology. All around the world scientists use CRISPR-Cas9 in
various branches of biology and medicine — not least to try to develop
novel therapeutic strategies to treat a range of diseases.
I am honoured and touched to receive these prestigious awards. This is tremendous recognition for me and my team, and also for fundamental research in microbiology, genetics and biochemistry.
But let’s come back to that a little later and start from the
beginning. French-born Charpentier is a microbiologist, biochemist and
geneticist — i.e. a researcher who with molecular biological methods
studies bacteria and interactions with their host. After completing a
Ph.D. at the prestigious Pasteur Institute in Paris and postdoctoral
positions in a series of research environments in the United States,
including The Rockefeller University, she headed up her own research
team at the University of Vienna in 2002. Six years later she was
offered and accepted a group leader position at the Laboratory for
Molecular Infection Medicine Sweden (MIMS) at Umeå University.
“I
was and remain very passionate about the MIMS concept at Umeå
University. It focuses on a very high level of education and research,
with regard to both high-quality research and insight into how to
promote fundamental research and the education of postgraduate students
in the long term. The concept also takes into account that good research
takes time and requires good working conditions in which a community
pools its energy and the administrative burden is small. I appreciate
that The Nordic EMBL Partnership for Molecular Medicine gave me the
academic freedom I needed.”
Test new ideas
For international recruitment of the best young researchers, MIMS
uses the model and strategy so successfully developed by EMBL. The model
implies that the young researchers, directly after their recruitment,
can set up their own lab with support for 4–5 PhD students and postdocs,
and gain access to advanced infrastructure and equipment. This is
combined with mentorship, unique opportunities for collaborations within
the multidisciplinary
Umeå Centre for Microbial Research (UCMR) and external evaluation, where also EMBL participates.
With secure funding through MIMS, Charpentier could start and continue her research projects and test new ideas.
“In
2008, I travelled back and forth between Austria and Sweden to set up
my lab at MIMS at Umeå University,” she says. “These trips also became
an opportunity for me to think through what I wanted my laboratory to
focus on over the next years: where I was in my research, what research
track I wanted to continue on and which new projects I wanted to pick
up. It was during one of those trips that I realised my lab should focus
more on the small RNAs that have a regulatory function in the cell — in
particular, a small RNA molecule that later became known as tracrRNA. I
decided to decipher its potential function in something called CRISPR.”
At the time, CRISPR was of interest primarily to microbiologists and was not particularly associated with genetic engineering. In fact, CRISPR, which stands for Clustered Regularly Interspaced Short Palindromic Repeats, comes from bacterial and archaeal (another kind of single-celled organism) genetics, that is, they were detected in the DNA of the microorganisms. These tiny DNA snippets have been known since the 1980s, but for a long time scientists thought that they were a form of “junk DNA” — i.e. DNA without information. Over the past decade, however, that view has been revised.
Infectious diseases remain the second most common cause of mortality worldwide and there are serious concernsabout the increasing problem of antiobiotic resistance. Therefore, we need to better understand how bacteria function and evolve.
During 2005, some scientists suspected that CRISPR was part of
bacteria’s defence against external attack, and in 2007 it became clear
that it actually is an adaptive immune system in bacteria: in other
words, a defence with the ability to remember an invading virus and thus
be better equipped against future attacks. The notion that bacteria
could have adaptive immune systems was previously unknown. For
Charpentier, the new findings were of huge interest.
“The goal of
my lab’s research was to understand an infection process from the
bacteria’s perspective: how they survive, adapt, protect themselves,
multiply and cause disease,” she says. “CRISPR relates to how bacteria
defend themselves against invaders such as viruses by targeting the
invader’s genome. Thus CRISPR influences the bacteria’s ability to cause
diseases or become resistant to infections.”
Came to Umeå
In early 2009, Charpentier began her new job as a group leader at MIMS at Umeå University. In the years that followed, in collaboration with groups in other parts of the world, her research team discovered more and more details about CRISPR sequences, the enzyme and other molecular components involved in the process. Together, these substances form a complex entity that finds and disables virus DNA by simply cutting it apart. The scissors are the Cas9 protein, and the small address label that ensures that the cut happens in the right place is made up of RNA. Bacteria can produce this RNA as needed — thus adapting their immune systems to scout for new viruses.
It quickly became clear to Charpentier that the CRISPR-Cas9 system was beginning to look like a potential tool for gene modification. By replacing the small RNA address label, you should be able to get the scissors to cut exactly where you want, virtually anywhere in the DNA.
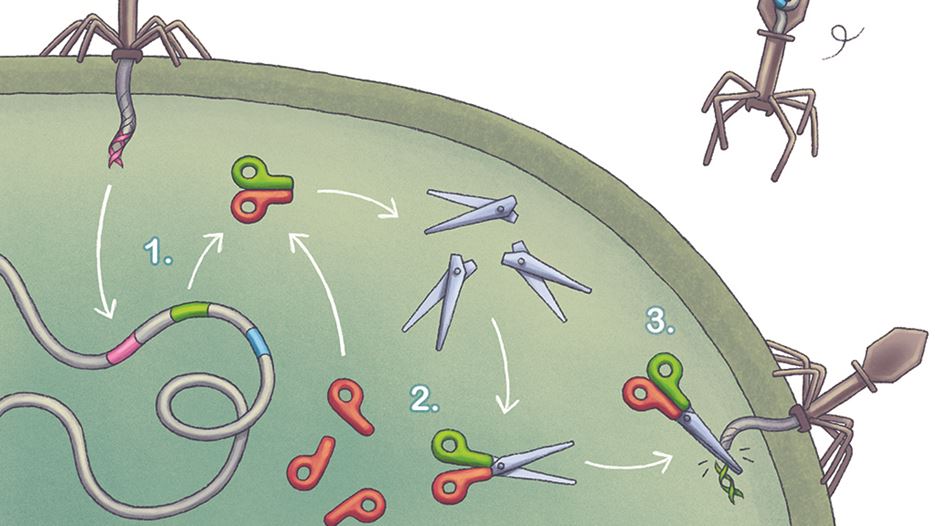
CRISPR-Cas9
Emmanuelle Charpentier and her team discovered the CRISPR-Cas9 mechanism that withstands virus attacks in bacterial immune systems. CRISPR-Cas9 consists of the following:
The CRISPR-Cas9 mechanism in bacteria illustrated here with two scissor blades representing the Cas-9 enzyme – CRISPR-RNA in green, tracrRNA in red. Together, they recognise and neutralise viral DNA upon attack (right).
- CRISPR DNA sequences (CRISPR stands for Clustered Regularly Interspaced Short Palindromic Repeats) are specific for each virus and encode unique RNA sequences. This sequence is the memory of virus DNA. One RNA molecule (the green handle) is called CRISPR RNA. It contains the memorised viral sequence used to recognise a new virus attack.
- Together with a second small RNA, tracrRNA (trans activating crRNA) (handle in red), the pair of RNAs guide the Cas9 enzyme (both scissor blades) to the corresponding DNA sequence.
- CRISPR-Cas9 (complete scissors) recognises the viral DNA during a new virus attack and cleaves and neutralises the intruding DNA to prevent a new infection. (Publications: Nature (2011) 471: 602-607, Science (2012) 337: 816-821).
Illustration: Linnea Holmström Ljung
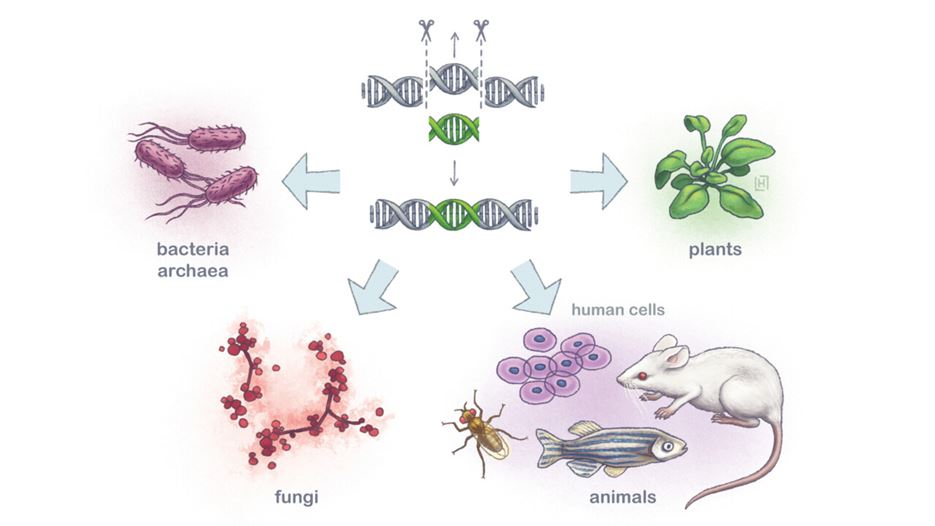
The CRISPR-Cas9 system (scissors) has been used for the genetic modification of cells in virtually all types of living organisms. The system recognises a precise sequence to be deleted or replaced with an alternative DNA sequence. Harmful genes can be silenced or replaced and individual mutations can be corrected.
Illustration: Linnea Holmström Ljung
“We realised that it ought to work and that, if it did, it could
be a very simple and flexible tool,” says Charpentier. “You only need to
design a new RNA sequence till the tool — a relatively simple matter.”
Rapid succession
Charpentier’s research group published an article in the journal
Science in August 2012, describing the targeting and cleavage of DNA by
CRISPR-Cas9. Since then, things have taken off. In rapid succession,
this new tool has been applied and developed across a variety of
research areas and practical contexts. For example, not only can CRISPR
cut DNA, it can also be applied to replace one gene sequence with
another.
“I had no doubt that it would work and had even
predicted earlier on that the system could be harnessed to treat human
genetic disorders. I have always performed my research at a basic level
but always keeping a perspective toward a possible exploitation of my
findings for potential benefit to human therapeutics. The Swiss-based
company CRISPR Therapeutics, which I co-founded together with Shaun Foy
and Rodger Novak, now focuses on this area. Suddenly, everyone wanted to
get involved in testing and applying the new tool. The new technology
was quickly adopted and readily applied by the scientific community,
confirming that CRISPR-Cas9 is useful in multiple cell types, including
human cells,” says Charpentier.
Most important academic achievements
Following the 2012 publication in Science, interest among genetic
researchers exploded and already by the first half of 2013, CRISPR-Cas9
was being used in labs around the world to alter genes in everything
from bacteria, yeast and plants to zebrafish, fruit flies, mice, rats
and human cells. In August 2013, the journal Science reported a “CRISPR
craze,” and at the end of the year, CRISPR-Cas9 made it onto both
Science’s and Nature’s top-ten lists of the most important academic
achievements in the past year.
“CRISPR-Cas9 is not the first tool
for genetic engineering, but it is simpler and more versatile than
earlier approaches such as ‘zinc fingers’ and ‘TALENs’,” Charpentier
explains. “The difference in versatility lies mainly in the fact that
the existing technologies are dependent on proteins as address labels,
whereas CRISPR-Cas9’s label is an RNA snippet. Customising a new protein
is a much more cumbersome process than producing an RNA sequence.”
With CRISPR-Cas9, gene therapies may be developed for a large number of diseases with genetic causes for which there is currently no treatment or where treatment could be much more effective.
CRISPR-Cas9 is like a Swiss army knife for genetic engineering — it
has been used in all types of living cells, and it can be programmed to
accurately cut and paste exactly where scientists want in the genome. It
is as though genetic engineering has been given a counterpart to the
computer function of “Find & Replace.” CRISPR-Cas9 has already
become important in a series of different research areas, but it is its
potential in medicine that has attracted the most attention.
“With
CRISPR-Cas9, gene therapies may be developed for a large number of
diseases with genetic causes for which there is currently no treatment
or where treatment could be much more effective,” says Charpentier.
Examples include haemophilia, sickle-cell disease, Pompe disease,
Huntington’s disease and cystic fibrosis.
Used in HIV and malaria research
CRISPR-Cas9 may also enable the development of new treatments for cancer and severe infectious diseases. For example, the tool is already being used in HIV and malaria research.
But research takes time. Despite the tremendous response in the
research community, it will be some time before the new tool can be used
in human medical treatment. Researchers must ensure there are no
significant “off-target effects;” in other words, cases where CRISPR
navigates incorrectly in the cell and manipulates the wrong genes.
One
potential route for treatment might be to take a cell sample from the
patient and correct the pathogenic gene in a cell culture in the lab.
The cured cells can then be grafted back into the patient — after
checking that CRISPR-Cas9 has carried out its job without any hitches.
In addition to new medical treatments, the new technology is expected to
be of great importance for the food industry. Another use is animals
research for testing purposes.

Emmanuelle Charpentier had a special visit from King Carl Gustaf XVI in her lab at MIMS in 2015. ImageJohan Gunséus
“Today we often use so-called transgenic animal models; in other
words, animals that have certain genetic changes,” says Charpentier.
“But making these changes is often a timeconsuming process. When I was a
postdoctoral researcher in New York, I came into contact with animal
research and created transgenic mouse models. I was amazed to see how
much time scientists had to devote to implement the genetic changes, and
how little time they got, in the end, to study their animal model and
draw their conclusions. With CRISPR, that changes. Scientists who are
working at the cutting edge are already up-and-running and using it.”
“CRISPR-Cas9
is on its way to transforming the biotechnology and medical landscapes.
The technology is very versatile and multiple versions of CRISPR-Cas9
have now been developed to target genomes and their expression in
various ways. There is still a lot of work to be done but the technology
has great potential for translation into gene medicines for the
treatment of certain human genetic disorders. However, we will have to
wait a couple of years before the first products are progressing in
early clinical trials. Other future directions include the development
of CRISPR-Cas9-technology for the treatment of other types of human
disease such as cancer and infectious diseases.”
The research at MIMS focuses on molecular infection mechanisms in various pathogenic microorganisms. The understanding generated is also expected to result in the development of alternative antimicrobial strategies. In addition, the understanding of CRISPR-Cas9 obtained by Charpentier’s research group will probably help to develop alternative treatment methods for infectious diseases and prevent antibiotic resistance in certain areas.
“Infectious diseases remain the second most common cause of mortality worldwide and there are serious concerns about the increasing problem of antibiotic resistance. Therefore, we need to better understand how bacteria function and evolve. CRISPR-Cas9 can benefit our understanding of the cellular and molecular mechanisms underlying bacterial, viral and other types of infectious disease, and point towards new pathways for the development of novel antimicrobial therapies. Investigations are ongoing to evaluate the possibility that CRISPR-Cas9 could be harnessed to treat infectious diseases.”
Raises ethical questions
A powerful new tool in gene engineering also raises ethical questions and concerns. How can we ensure that gene manipulation isn’t used in a way that harms humanity? In April 2015, Chinese scientists reported that they had edited the genetic make-up of human embryos using CRISPR-Cas9. The trials were carried out on defective single-cell embryos from an IVF clinic, which could never have become babies, but the news has reignited the debate about regulating genetic engineering.
The understanding will probably help to develop alternative treatment methods for infectious diseases and prevent antibiotic reistance in certain areas.
Charpentier thinks it is good that ethical issues are receiving so
much attention. At the same time, she points out that there have long
been established principles to comply with, because it has been possible
for four decades to modify DNA using genetic engineering. These rules
are just as relevant to CRISPR-Cas9 as they have been to all the
previous genetic engineering tools. One of the underlying principles has
been that the human genome should not be edited in germline cells, so
that changes introduced to treat an individual patient, for example,
would not be passed on to future generations.
“Therapeutics
should be developed according to very high safety standards and are most
important in situations where there are no similarly effective or
readily available treatment options. Scientists, clinicians, patients
and the industry as a whole, as well as experts on ethical and related
legal questions, need to have an open dialogue on the risks and benefits
of precise gene-editing technologies in germline modification.”
Continued to be affiliated at Umeå
In 2015, she accepted a position as a scientific member of the Max
Planck Institute for Infection Biology in Berlin, Germany. But she
continued to be affiliated at Umeå for a long time as a visiting
professor. Unlike many other colleagues, Charpentier has been keen not
only to change her environment but also occasionally to change her
research track during her career.
“Changing your subject is
certainly risky but also very instructive, just as it’s stimulating and
challenging to move between countries and research environments. It has
been important to me. It stimulates you to reflect, question and find
the right approach to new questions, and you often think more freely
when you come from the outside. You also get the chance to work with new
colleagues.”

“I was and remain very passionate about the MIMS concept at Umeå University. It focuses on a very high level of education and research, with regard to high-quality research and good insight into how to promote fundamental research and the education of postgraduate students in the long run.” ImageHallbauer&Fioretti
“For this reason it has been an amazing experience to be involved in and to establish a new research field,” she explains.
“CRISPR has brought together talented researchers from various fields — bacteriology, molecular biology, genetic engineering, etc. — and together we are challenged to think in completely new ways and explore the unknown from scratch. It’s incredibly exciting to work in such a vital field, where there isn’t yet any dogma, and where one really has to be creative.”
Revenge for microbiology
But she also sees the CRISPR fever as something of a revenge for
microbiology, which, in the shadow of high-prestige areas of biology
such as cancer research and neurology, tends to be a little neglected.
“Microbiology
is often seen as a rather old field in which most aspects have been
researched and the field has now been exhausted. In fact, this is where
there is the most work left to do. Infectious diseases remain the second
most common cause of mortality worldwide and there are serious concerns
about the growing problem of antibiotic resistance. Therefore, we need a
better understanding of how bacteria function and evolve. As a tool to
treat human genetic diseases, CRISPR has bridged boundaries between
different areas of biology and shown once again how important
discoveries in microbiology can be!”
This article was first published in 2015 and updated in 2019. Since then, Emmanuelle Charpentier has been awarded the Nobel Prize in Chemistry 2020.
